Merck Animal Health Expands Voluntary Recall with Four Additional Lots of BANAMINE® / BANAMINE®-S (flunixin meglumine injection) in the U.S.
All images courtesy FDA/Merck
From the Federal Drug Administration:
Summary
- Company Announcement Date:
- FDA Publish Date:
- Product Type:
- Animal & Veterinary
Animal Drugs - Reason for Announcement:
-
Potential presence of particulate matter
- Company Name:
- Merck Animal Health, known as MSD Animal Health outside of the United States and Canada, a division of Merck & Co., Inc.
- Brand Name:
-
Merck Animal Health
- Product Description:
-
Banamine; Banamine-S
Company Announcement
RAHWAY, N.J., Sept. 29, 2023 – Merck Animal Health, known as MSD Animal Health outside of the United States and Canada, a division of Merck & Co., Inc., Rahway, N.J., USA (NYSE:MRK), is voluntarily recalling four additional batches of BANAMINE® / BANAMINE®-S (flunixin meglumine injection) 50 mg/mL in the United States, used for injection in cattle, swine and horses due to the presence of particulate matter. These batches are in addition to the BANAMINE® / BANAMINE®-S recall of three batches dated Sept. 1, 2023, due to the presence of particulate matter. BANAMINE® / BANAMINE®-S (flunixin meglumine injection) is a prescription product in the U.S.
Particulates were observed during routine quality testing and reviews for the following additional batches:
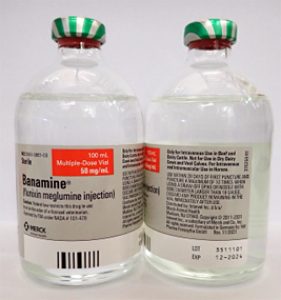
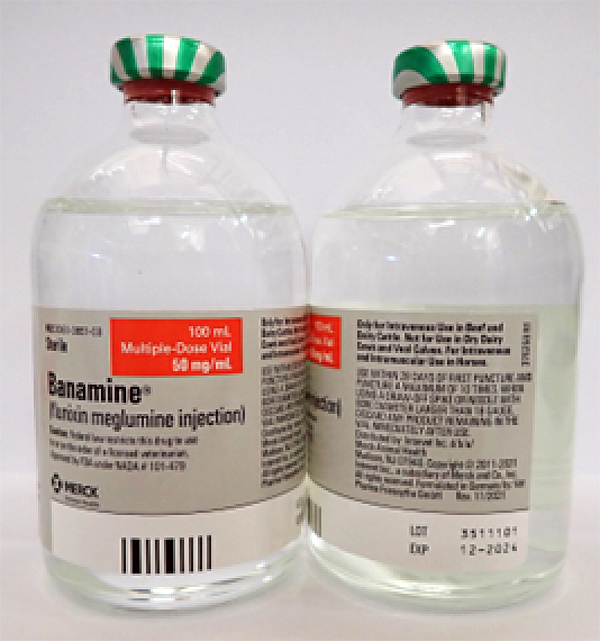
- BANAMINE 100mL, UIN 065474, NDC 00061-0851-03, Batch 3511101, exp Dec. 2024
- Distribution dates: May 16, 2023, to August 8, 2023
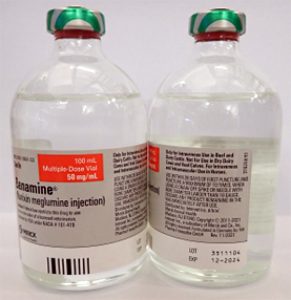
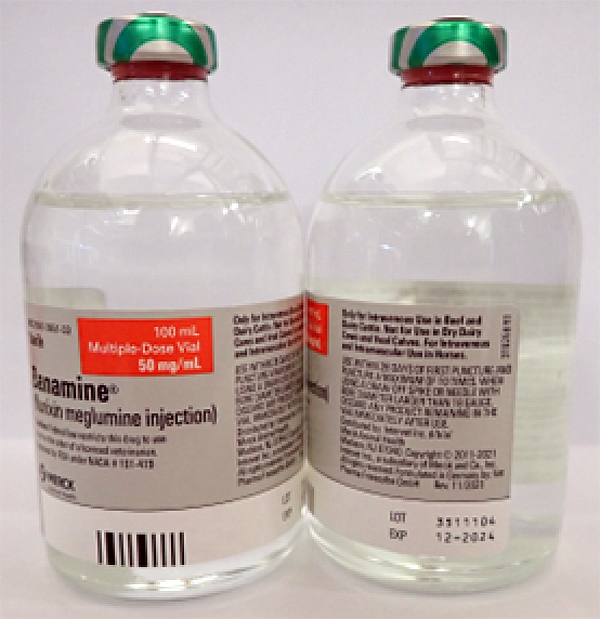
- BANAMINE 100mL, UIN 065474, NDC 00061-0851-03, Batch 3511104, exp Dec. 2024
- Distribution dates: August 4, 2023, to August 17, 2023
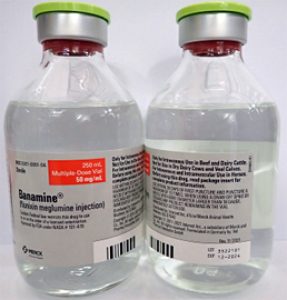
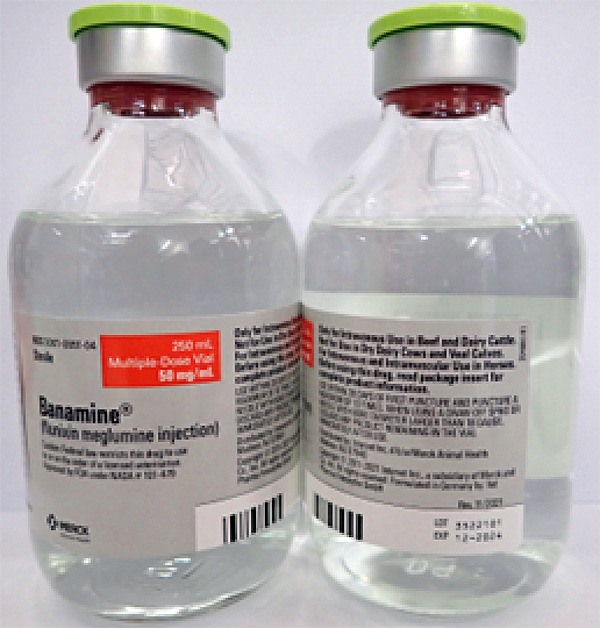
- BANAMINE 250mL, UIN 065476, NDC 00061-0851-04, Batch 3522101, exp Dec. 2024
- Distribution dates: July 14, 2023, to August 17, 2023
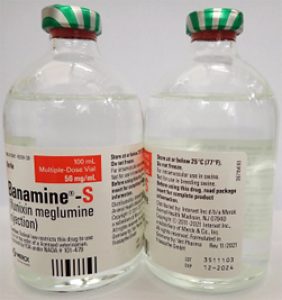
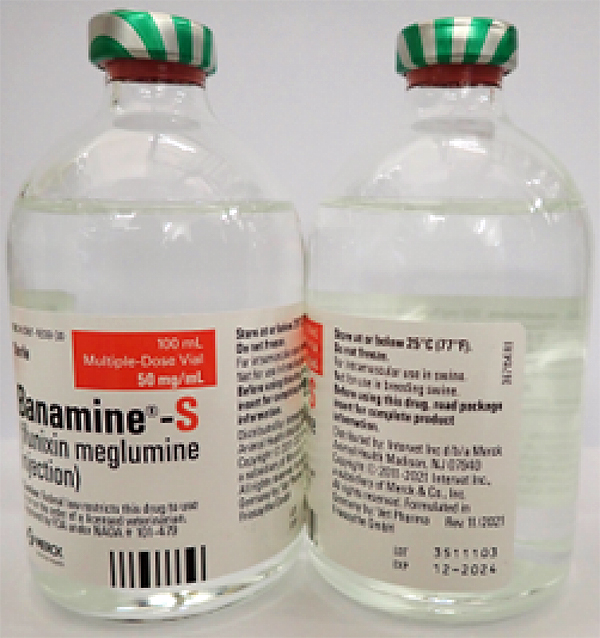
- BANAMINE-S 100mL, UIN 065477, NDC 0061-1838-30, Batch 3511103, exp Dec. 2024
- Distribution dates: May 3, 2023 to August 16, 2023
A photo of the bottle of each batch number (lot number) that is part of the recall can be found at the end of this press release. The lot number (LOT) and expiry date (EXP) is located in the bottom right portion of the bottle label.
The administration of an injectable product that contains particulate matter may result in local irritation, swelling or infection in response to the foreign material. After intravenous administration in large animals, such as cattle or horses, particulate matter could travel to the lungs which could result in local tissue damage.
Flunixin meglumine is a potent, non-narcotic, nonsteroidal, analgesic agent with anti-inflammatory and antipyretic activity. It is approved in the US only for intravenous use in beef and dairy cattle, for intravenous and intramuscular use in horses and for intramuscular use in swine.
Customers who have received BANAMINE® and BANAMINE®-S from the batches being recalled should stop using the products and refer to their recall letter for product return instructions. Merck Animal Health is working with our distributor partners to ensure that unused product is no longer in distribution or with customers. We are notifying our distributors and customers directly and are arranging for the return of all recalled product.
Consumers with technical questions regarding this recall should call 1-800-221-3573 (Monday through Friday 8 a.m. – 5 p.m. CDT). Customers who may need to arrange return of product should contact their point of purchase.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA at 1-888-FDA-VETS or online at http://www.FDA.gov/reportanimalae.
This recall is being made with the knowledge of the Food and Drug Administration.
The health and well-being of animals is the foremost priority at Merck Animal Health. We place the utmost emphasis on product quality at every step in the manufacturing and supply chain process.
About Merck Animal Health
At Merck, known as MSD outside of the United States and Canada, we are unified around our purpose: We use the power of leading-edge science to save and improve lives around the world. For more than a century, we’ve been at the forefront of research, bringing forward medicines, vaccines and innovative health solutions for the world’s most challenging diseases. Merck Animal Health, a division of Merck & Co., Inc., Rahway, N.J., USA, is the global animal health business of Merck. Through its commitment to The Science of Healthier Animals®, Merck Animal Health offers veterinarians, farmers, producers, pet owners and governments one of the widest ranges of veterinary pharmaceuticals, vaccines and health management solutions and services as well as an extensive suite of connected technology that includes identification, traceability and monitoring products. Merck Animal Health is dedicated to preserving and improving the health, well-being and performance of animals and the people who care for them. It invests extensively in dynamic and comprehensive R&D resources and a modern, global supply chain. Merck Animal Health is present in more than 50 countries, while its products are available in some 150 markets. For more information, visit www.merck-animal-health.com and connect with us on LinkedIn, Facebook, Twitter and Instagram.
Forward-Looking Statement of Merck & Co., Inc., Rahway, N.J., USA
This news release of Merck & Co., Inc., Rahway, N.J., USA (the “company”) includes “forward-looking statements” within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company’s management and are subject to significant risks and uncertainties. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of the global outbreak of novel coronavirus disease (COVID-19); the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company’s Annual Report on Form 10-K for the year ended December 31, 2022 and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC’s Internet site (www.sec.gov).
###
All images courtesy FDA/Merck

Recently Added
- Making History December 23, 2024
- 2025 AQHA Convention Schedule Released December 23, 2024
- From The Publisher – The Horse Capital Of The World December 23, 2024
- EC Wreath Photo of the Day – Fancy This Lope December 23, 2024
- EC Wreath Photo of the Day – Illini Saige December 22, 2024
- Low-NSC Diets: Not for Every Horse December 21, 2024
- On The Cover – VS The Fireman December 21, 2024
- EC Photo of the Day – Merry Christmas from Elvada Farm December 21, 2024
- EC Video of the Day – Smile! It’s Friday! December 20, 2024
- Sun Circuit Debuts English Versatility Class in 2025 December 20, 2024
Archives
Sign In
Equine Chronicle ® All Rights Reserved. Copyright © 2024
4727 NW 80th Ave. • Ocala, FL 34482 • 352 369 1104 • FAX 352 369 1521
Privacy Policy | Questions, please contact The Equine Chronicle
-